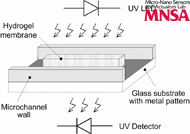
A microfluidic system to sense chemical and biological analytes using membranes dissolvable by the analyte is demonstrated. The scheme to detect the dissolution of the membrane is based on the difference in optical absorption of the membrane and the fluidic sample being assayed. The presence of the analyte in the sample chemically cleaves the membrane and causes the sample to flow into the membrane area. This causes a change in the optical absorption of the path between the light source and detector. A device comprising the microfluidic channels and the membrane is microfabricated using liquid-phase photopolymerization. A light emitting diode (LED) and a detector with an integrated amplifier are positioned and aligned on either side of the device. The state of the membrane is continuously monitored after introducing the sample. The temporal dissolution characteristics of the membrane are extracted in terms of the output voltage of the detector as a function of time. This is used to determine the concentration of the analyte. The absorption spectra of the membrane and fluidic sample are studied to determine the optimal wavelength that provides the maximum difference in absorbance between the membrane and the sample. In this work, the dissolution of a poly(acrylamide) hydrogel membrane in the presence of a reducing agent (dithiothreitol—DTT) is used as a model system. For this system, with 1 M DTT, complete membrane dissolution occurred after 65 min.